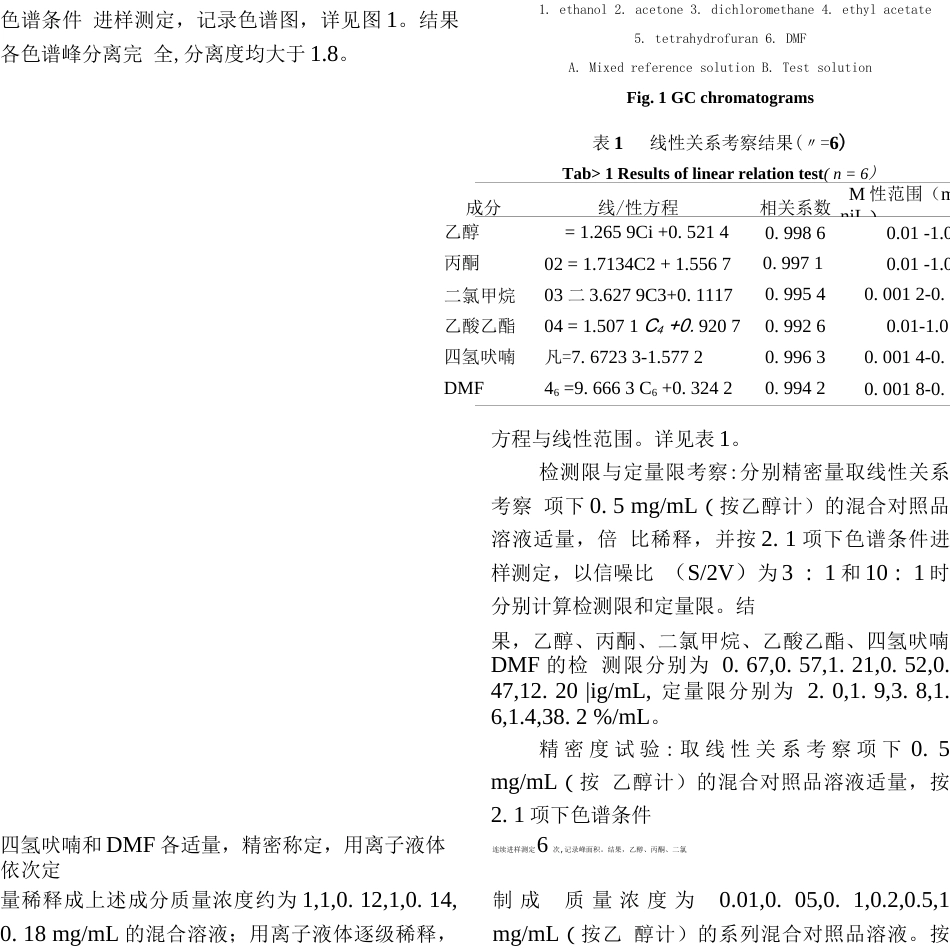
doi:10.3969/j.issn.1006-4931.2021.16.022离子液体■顶空气相色谱法同时检测甲苯磺酸索拉非尼原料中6种有机溶剂残留张轶华I,于洲2△,王柳*高燕霞I(1.河北省药品医疗器械检验研究院,河北石家庄050200;2.石家庄四药有限公司,河北石家庄052165)摘要:目的建立可同时检测甲苯磺酸索拉非尼原料中有机溶剂残留的离子液体-顶空气相色谱法。方法色谱柱为HP-5毛细管色谱柱(30mxO.32mmJpirn),载气为氮气,检测器为火焰离子化检测器,检测器温度为220°C,进样口温度为200°C,程序升温,溶剂为1-丁基-3-甲基咪喳四氟硼酸盐离子液,进样方式为顶空进样。结果乙醇、丙酮、二氯甲烷、乙酸乙酯、四氢吠喃和N,N—二甲基甲酰胺(DMF)质量浓度分别在0.01〜l.0mg/mL,0.01〜1.0mg/mL,0.0012〜0.12mg/mL,0・01〜1.0mg/mL,0・0014〜0.14mg/mL,0.0018〜0.18mg/mL(r均大于0.992)范围内与峰面积线性关系良好;检测限分别为0.67,0.57,1.21,0.52,0.47,12.20pig/mL,定量限分别为2.0,l.9,3.8,l.6,l.4,38.2jjig/mL;精密度试验的RS〃值分别为0.53%,0.50%,3.25%,0.85%,1.71%,3.82%"=6);平均加样回收率分别为93.33%,98.78%,91.13%,94.48%,90.93%,90.39%,RSD分别为1.91%,0.66%,1.07%,0.52%,1.27%,1.16%(卫=9)。结论采用离子液体作为溶剂,操作简便、检测限低、结果准确、重复性好,可用于多种溶剂残留的同时测定,扩展了顶空气相色谱法在药物有机溶剂残留测定中的应用范关键词:原料药;甲苯磺酸索拉非尼;残留溶剂测定;离子液体;顶空气相色谱法SimultaneousDeterminationofSixOrganicSolventResiduesintheActivePharmaceuticalIngredientsofSorafenibTosylatebyIonicLiquid一HeadspaceGasChromatographyZHANGYihua1,YUZhou2,WANGLiu1,GAOYanxia1(1.HebeiInstituteforDrugandMedicalDeviceControl,Shijiazhuang,Hebei,China050200;2.ShijiazhuangNO.4PharmaceuticalCo.,Ltd..Shijiazhuang,Hebei,China052165)中图分类号:R917文献标志码:A文章编号:1006一4931(2021)16-0085一04Abstract:ObjectiveToestablishanionicliquid-headspacegaschromatographymethodforthesimultaneousdeterminationoforganicsolventresiduesintheactivepharmaceuticalingredients(API)ofsorafenibtosylate.MethodsThechromatographiccolumnwasHP-5capillarycolumn(30mx0.32mm,1|jim),thecarriergaswasnitrogen,thedetectorwasflameionizationdetector(FTD)withlhetemperatureof220°C,theinjectionporttemperaturewas200°Cwithtemperature-programmed,thesolventwas1-butyl-3-methylimi-dazoliumtetrafluoroborateionicliquid,andthesamplingmethodwasheadspaceinjection.ResultsThelinearrangesofethanol,acetone,dichloromethane,ethylacetate,tetrahydrofuranandN,N-dimethylformamide(DMF)were0.01-1.0mg/mL,0.01-1.0mg/mL,0.0012-0.12mg/mL,0.01-1.0,0.0014-0.14mg/mL,0.0018-0.18mg/mL(r>0.992),theirlimitsofdetection(LOD)were0.67,0.57,1・21,0・52,0.47and12.20|Jig/mL,respectively,andthetheirlimitsofquantitation(LOQ)were2.0,1.9,3.8,1.6,1.4and38.2|JLg/mL,respectively.TheRSDsoftheirprecisiontestswere().53%,0.50%,3.25%,0.85%,1.71%and3.82%(n=6),respectively.Theaveragerecoveriesofethanol,acetone,dichloromethane,ethylacetate,tetrahydrofuranandDMFwere93.33%,98.78%,91.13%,94.48%,90.93%,90.39%withRSDsof1.91%,0.66%,1.07%,0.52%,1.27%and1.16%(〃=9),respectively.ConclusionThemethodwithionicliquidassolventhastheadvantagesofsimpleoperation,lowdetectionlimit,accurateresultsandgoodreproducibility,whichcanbeusedforthesimultaneousdeterminationofvarioussolventresidues,andfurtherexpandstheapplicationscopeofheadspacegaschromatographyinthedeterminationoforganicsolventresiduesindrugs.Keywords:activepharmaceuticalingredients;sorafenibtosylate;determinationoforganicsolventresidues;ionicliquid;headspacegaschromatography甲苯磺酸索拉菲尼属新型多靶点抗肿瘤药...